
CMV and its challenges
Watch a discussion of current unmet needs in the management of CMV post-transplantation
Featured in this video:
• Mr Colin Wilson (Consultant Transplant and Hepatobiliary Surgeon, Newcastle Hospitals)
• Dr Adnan Sharif (Consultant Nephrologist with Special Interest in Transplantation, University Hospitals Birmingham)
• Dr Sowsan Atabani (Consultant Virologist, UK Health Security Agency and University Hospitals Birmingham)
Book a meeting
Click this link to discuss the information on this page further with a Takeda representative.
What is cytomegalovirus (CMV)?
CMV is a common beta virus that is a member of the herpes virus family.1 The prevalence in UK adults is estimated to be 50–60%.2
In immunocompetent individuals, the initial infection is generally asymptomatic but can present as flu-like symptoms.3 After the initial infection, the virus has the ability to establish lifelong latent infection.4 In immunocompromised individuals (such as those receiving transplants), new infection, reinfection with a different virus strain, or reactivation of the latent virus can result in serious complications and death.3,5
Consequently, CMV is one of the most common infections experienced by transplant recipients:
| Estimated incidence rate of 16–56% in solid organ transplant (SOT) recipients5 | Estimated incidence rate of 30–70% in haematopoietic stem cell transplant (HSCT) recipients6 |
CMV infection can progress to tissue-invasive disease and may alter the immune system: poor outcomes related to the direct and indirect effects of CMV7,8

Current challenges in post-transplant CMV
Managing vulnerable transplant patients can be a balancing act. Finding the right level of immunosuppression and treating complications such as emergent CMV infection, while trying to avoid additional treatment burden, adds complexity to post-transplant management.12,13
CMV considerations post-transplant
| HSCT | SOT |
|---|---|
|
|
Treating refractory CMV infections after transplant can be complicated by safety and tolerability issues. Experts call for new treatment options with specific focus on reducing toxicity without compromising potency.12,13,17
Identifying the risk factors of refractory CMV could support appropriate management:19–22
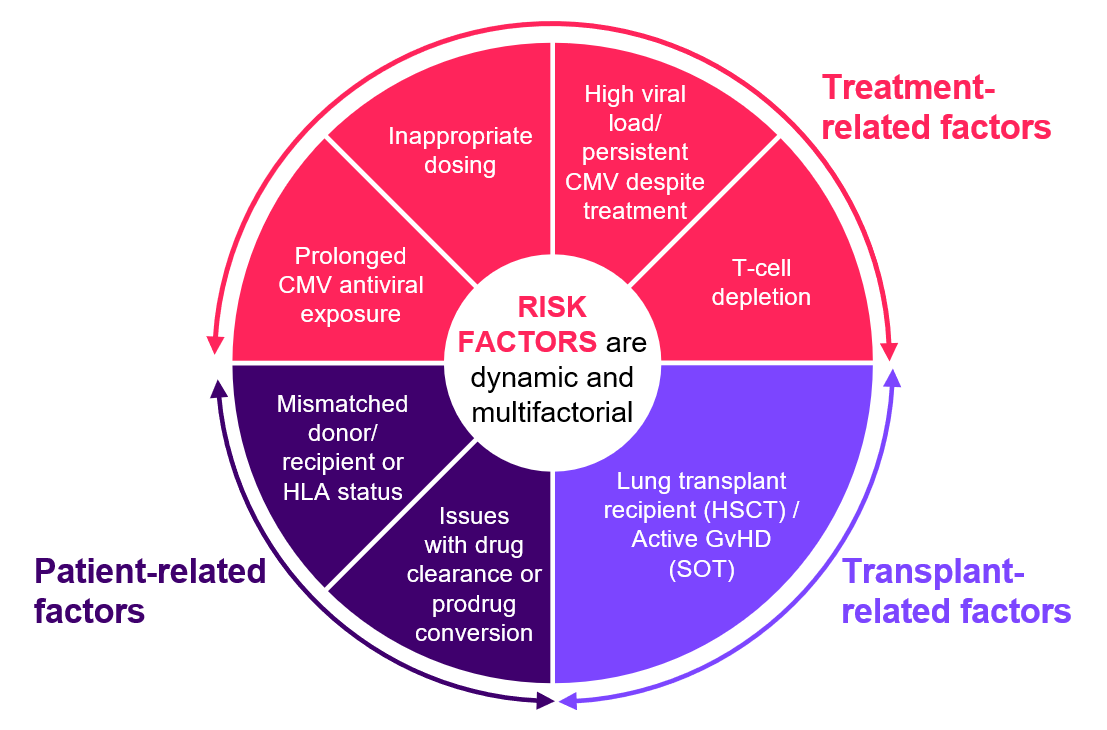
CMV = cytomegalovirus; GvHD = graft-versus-host disease; HLA = human leukocyte antigen.
References:
- Crought T, Khanna R. Clin Microbiol Rev. 2009;22(1):76–98.
- NHS Blood and transplant. Factsheet. CMV negative blood components. 2018.
- Azevedo LS, et al. Clinics (Sao Paulo). 2015;70(7):515–23.
- Meesing A, Razonable RR. Drugs. 2018;78(11):1085–103.
- Styczynski J. Infect Dis Ther. 2018;7(1):1–16.
- Cho SY, et al. Int J Mol Sci. 2019;20(11):2666.
- Emery VC, et al. Lancet. 2000;355:2032–6.
- Jorgenson MR, et al. Transpl Infect Dis. 2019;21(3):e13080.
- Ljungman P, et al. Clin Infect Dis. 2017;64:87–91.
- Stern M, et al. Transplantation. 2014;98:1013–8.
- Robin C, et al. BMC Infect Dis. 2017;17:747.
- Kotton CN, et al. Transplantation. 2018;102:900–31.
- Ljungman P, et al. Lancet Infect Dis. 2019;19(8):e260–72.
- Yong MK, et al. BBMT. 2017;23(11):1961–7.
- Xiao Y, et al. Int J Med Sci. 2014;11(6):652–7.
- Hakimi Z, et al. Curr Res Transl Med. 2018;66(4):95–101.
- Khawaja F, et al. Clin Microbiol Infect. 2023;S1198-743X(22)00348–2.
- Hakimi Z, et al. Transpl Infect Dis. 2017;19(5):doi: 10.1111/tid.12732. Epub 2017 Jul 21.
- Fisher CE, et al. Clin Infect Dis. 2017;65:57–63.
- Liu J, et al. Clin Microbiol Infect. 2015;21(12):1121.e9–15.
- El Chaer F, et al. Blood. 2016;128:2624–36.
- Sandkovsky U, et al. Transplant Proc. 2018;50(10):3763–8.
Click on the links below to learn more:
An overview of LIVTENCITY▼® (maribavir)


