Androgen use in patients with hereditary angioedema
Latest Evidence on Androgen use in HAE:
Overview
Attenuated androgens, such as danazol, have been commonly used for long-term prophylaxis in hereditary angioedema (HAE) despite safety concerns and recommendations from the European Academy of Allergy & Clinical Immunology (EAACI) and World Allergy Organization (WAO) to use them as second-line treatments. In the UK, where these agents are not licensed for HAE treatment, they are still widely used.1-2 A 2018 national survey found that 45% (n=38) of HAE patients on long-term prophylaxis were using androgens, and interim results from a study published in 2024 showed similar usage rates.2-3 While effective in reducing attack frequency and severity, the long-term use of androgens is associated with significant adverse effects. Clinicians are advised to consider the safety profiles of modern prophylactic agents and to make individualised treatment decisions based on attack characteristics and patient preferences.1 Notably, only two small randomised controlled trials have investigated the efficacy of attenuated androgens in HAE.4
Here we discuss some of the outcomes of more up to date studies conducted in larger cohorts of patients of HAE, focussing on the safety and efficacy of androgens in current practice. These provide valuable insights into the benefits and risks of androgen therapy, highlighting the need for careful patient selection and monitoring.1
Safety of Androgens
Attenuated androgens are known to be associated with frequent and sometimes severe adverse outcomes. In the last couple of years there have been new studies characterising this further.5
A systemic literature review published in 2024 looking at androgen use and adverse health outcomes, found that the use of androgens was associated with a range of adverse outcomes, including weight gain, liver damage, menstrual irregularities, virilization, and cardiovascular risk factors such as elevated low-density lipoprotein cholesterol (LDL-C) and decreased high-density lipoprotein cholesterol (HDL-C) levels. The literature referenced included studies with a duration of androgen use spanning from 6 months to more than 30 years and identified that adverse effects were dose-related and often severe, leading to significant concerns among both patients and physicians regarding the long-term use of androgens.5 Of note, some of the larger studies in recent decades such as Fust et al, which involved 84 patients, reported a range of adverse effects, including weight gain, liver tumours, anxiety, altered libido, alopecia, and hypertension.8 These findings align with a study published in 2008, who additionally highlighted a high incidence of adverse effects such as virilization, menstrual irregularities, headache, depression, liver adenomas, myocardial infarction, and stroke in 188 patients. The severity of these side effects led to the discontinuation of danazol in a substantial proportion of patients.9 A 2016 study including 650 subjects, identified that weight gain, menstrual abnormalities, acne, liver damage, and hypertension were common and often severe. This study emphasised that the side effects frequently outweighed the benefits, particularly at higher doses.4
Particularly interesting was a recent study from the Milan and Padua angioedema centre which employed a retrospective cohort design spanning the years 1979 -2021, comparing the prevalence of comorbidities in patients with HAE to the general Italian population respectively. A total of 446 patients were studied and the key outcomes revealed a higher prevalence of comorbidities such as heart diseases (9.6% vs. 4.8%), acute myocardial infarction (5.6% vs. 1.4%), HCV infection (10.5% vs. 2.5%), and appendectomy (15.9% vs. 4.3%) in HAE patients compared to the general population, and an association between long-term prophylaxis with attenuated androgens and increased incidences of hypertension (22.8% vs. 10.8%), hypercholesterolemia (19.5% vs. 5.3%), diabetes mellitus (5% vs. 1.4%), hepatic angioma (4.4% vs. 0.7%), and focal nodular hyperplasia (2.5% vs. 0.4%).6
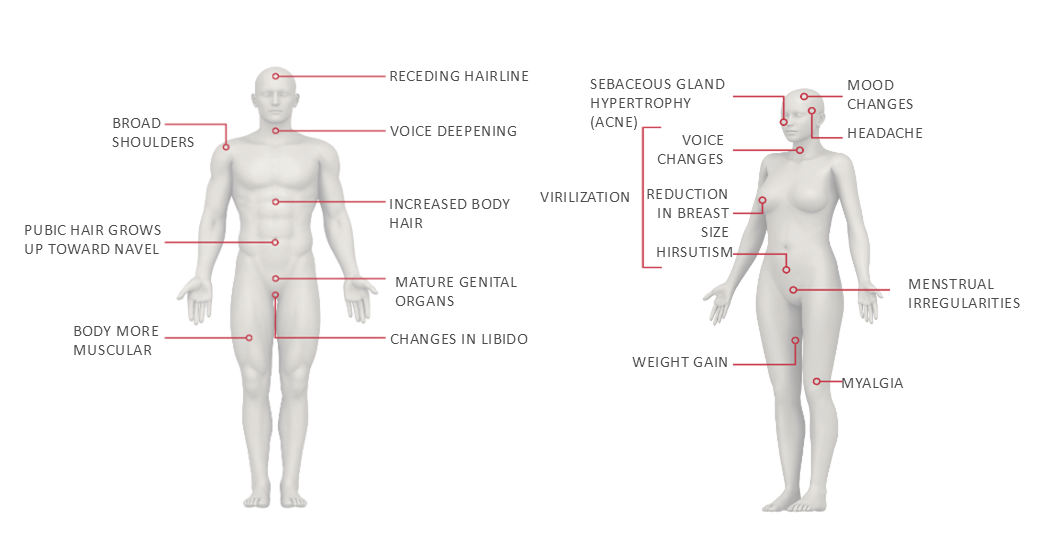
Common adverse effects associated with androgen therapy10-11
Efficacy of Androgens
The evidence of efficacy for androgens has been by and large based on cohort studies. A prospective, 6-year follow-up of two patient cohorts study evaluated the long-term efficacy of danazol in HAE patients over a six-year period, including German (n=45) and Hungarian (n=39) cohorts and found a significant reduction in mean attack frequency during the first year of treatment. However, the long-term efficacy varied between the two cohorts. Hungarian patients experienced an increase in subcutaneous and abdominal attacks over time, whereas German patients did not. The authors suggested that this discrepancy pointed to the possibility of genetic factors or baseline attack patterns influencing the long-term efficacy of danazol.8
A study published in 2008 conducted a retrospective analysis of 118 patients to examine the benefits and risks of long-term danazol treatment. This study also found that danazol significantly reduced the frequency and severity of attacks, with 45.8% of patients becoming symptom-free or experiencing very few attacks (1 attack or less per year). Despite its efficacy, the study highlighted a high incidence of adverse effects (weight gain, virilization, menstrual irregularities, headache, depression, and/or liver adenomas) occurred in 93 of the 118 patients and led to discontinuation of danazol therapy in 30 patients.9
Furthermore a study published in 2016 investigated the tolerability and effectiveness of androgens in a large cohort through a retrospective cross-sectional study using a web-based survey of 650 subjects. The study confirmed that androgens significantly reduced attack frequency and severity for 524 patients (24 down to 4 attacks/year, p<0.0001). However, there was substantial variability in their effectiveness, with many patients continuing to experience frequent attacks despite treatment. The study also reported frequent and severe side effects, suggesting that androgens should be used cautiously and at the lowest effective dose.4
Finally, a prospective study conducted over 21 months to assess the impact of standard care on angioedema in 103 HAE patients, including 41 patients on attenuated androgens. On average, patients on prophylaxis with attenuated androgens had 7.7 attacks/year lasting 1.47 days, those on tranexamic acid had 8.1 attacks/year lasting 1.59 days, and those without prophylaxis had 8.9 attacks/year lasting 1.68 days. However, it also noted that androgens did not completely eliminate attacks, indicating partial efficacy.7
Limitations of Available Data
The available data on androgen use for HAE is limited by retrospective study designs, variable sample sizes, follow-up periods, treatment regimens, inconsistent adverse effect reporting, and differences in patient populations, impacting generalizability and applicability to broader populations.6
Conclusion
Androgens are effective in reducing the frequency and severity of HAE attacks but come with significant adverse effects, leading to discontinuation in many cases. Limitations in current data highlight the need for more robust studies. Maurer et al. (2024) emphasize caution due to serious health outcomes.5-6 EAACI/WAO guidelines recommend modern agents like lanadelumab, berotralstat, and plasma-derived C1-esterase inhibitor as first-line options, with androgens as second-line. Careful patient selection, monitoring, and regular follow-up, including semi-annual blood and urine tests and annual liver ultrasounds, are essential for optimal HAE management.1
FAQ on Androgen Withdrawal
Disclaimer: These are suggestions developed in collaboration with an experienced clinician: Dr Sorena Kiani, Consultant Immunologist, Royal Free London NHS Foundation Trust. Each patient should be consulted after a review assessing if it is appropriate and agreeable for them to switch.
- How fast could androgens be stopped and what needs to be considered before you stop? The method of androgen discontinuation depends on the patient.
- My practice involves sometimes stopping androgens abruptly when there is an acute or serious side effect such as polycythaemia.
- The ideal situation, however, is to taper off androgens over a month to six weeks.
- There is a likelihood of recurrence of HAE attacks during this tapering period, which needs to be discussed with patients, and a plan of action put in place. This helps with patient expectations and compliance with the proposed management plan. It is useful at this point to remind patients of the benefits of stopping androgens.
- Patients should be encouraged to keep an attack diary to see if they become eligible for commissioned prophylaxis. I believe patients find this helps them to cope with their attacks, maybe because it documents their suffering and that in itself can be therapeutic, as well as giving them a chance of accessing modern prophylaxis.
- If possible, I recommend leaving a gap of 1 or 2 months before starting a new prophylactic medication but sometimes patients have too many attacks and are not able to manage with acute medications alone. A gap helps the patient to get through the period of transaminitis owing to androgen withdrawal. Some physicians have overlapped other prophylactic medications while tapering androgens, particularly in the United States and Europe, but this requires careful monitoring as combination of androgens with other medications increases the risk of adverse events.
- It is important to stress that androgens can cause adverse events even after discontinuation. The most common abnormality that can start after withdrawal of androgens and can persist for a few of months is transaminitis. This is usually mild to moderate, but some clinicians have noted changes in liver metabolism of other drugs in association with these modest levels of transaminitis. Therefore, it is strongly recommended that patients are monitored closely after androgen withdrawal, particularly if they are taking other medications which are metabolised by the liver.
- What investigations need to be done before stopping androgens?
- Bloods: FBC, LFTs, Lipid profile, U&Es
- Ultrasound of the liver if not done recently
- ECG if considering starting a modern prophylactic medication for HAE
In accordance with the EAACI/WAO guidelines it is recommended that patients undergo the above testing while on androgens.
EAACI/WAO, European Academy of Allergology and Clinical Immunology/World Allergy Organisation; ECG, Electrocardiogram; FBC, Full Blood Count; HAE, Hereditary Angioedema LFT, Liver Function Test; U&Es, Urea and Electrolytes.
References:
- Maurer M, Magerl M, Betschel S, Aberer W, Ansotegui IJ, Aygören-Pürsün E, et al. The international WAO/EAACI guideline for the management of hereditary angioedema - The 2021 revision and update. Allergy. 2022;77(7):1961-1990. doi:10.1111/all.15214.
- Yong PF, Coulter T, El-Shanwany T, Garcez T, Hackett S, Jain R, et al. A national survey of hereditary angioedema and acquired C1 inhibitor deficiency in the United Kingdom. J Allergy Clin Immunol Pract. 2023. doi: 10.1016/j.jaip.2023.04.035.
- Takeda Data on File: EXA/GB/FIR/009. 2024.
- Zuraw BL, Davis DK, Castaldo AJ, Christiansen SC. Tolerability and Effectiveness of 17-a-Alkylated Androgen Therapy for Hereditary Angioedema: A Re-examination. J Allergy Clin Immunol Pract. 2016;4(5):948-55.
- Maurer M, Pandey R, Lopez-Gonzalez L, Pedrosa M, Bloudek L, Gillard P. Adverse Health Outcomes and Patient and Physician Perspectives of Attenuated Androgen Use in Hereditary Angioedema. Presented at The European Academy of Allergy and Clinical Immunology (EAACI) Annual Congress, Valencia, Spain. May 31 - June 3, 2024.
- Zanichelli A, Senter R, Merlo A, Gidaro A, Janu VP, Cogliati CB, Cancian M. Comorbidities in Angioedema due to C1-inhibitor deficiency: an Italian survey. J Allergy Clin Immunol Pract. 2024. doi: 10.1016/j.jaip.2023.12.046.
- Zanichelli A, Vacchini R, Badini M, Penna V, Cicardi M. Standard care impact on angioedema because of hereditary C1 inhibitor deficiency: a 21-month prospective study in a cohort of 103 patients. Allergy. 2011;66(2):192-196.
- Füst G, Farkas H, Csuka D, Varga L, Bork K. Long-term efficacy of danazol treatment in hereditary angioedema. Eur J Clin Invest. 2011;41(3):256-262. doi:10.1111/j.1365-2362.2010.02402.x
- Bork K, Bygum A, Hardt J. Benefits and risks of danazol in hereditary angioedema: a long-term survey of 118 patients. Ann Allergy Asthma Immunol. 2008;100(2):153-161.
- Banerhi A, Sloane DE, Sheffer AL. Hereditary angioedema: a current state-of-the-art review, V: attenuated androgens for the treatment of hereditary angioedema. Ann Allergy Asthma Immunol. 2008;100(Suppl 2):S19-22.
- Riedl, MA. Critical appraisal of androgen use in hereditary angioedema: a systematic review, Ann Allergy Asthma Immunol. 2015;114:281-288.